12 Medical Release Form Best Practices
Are Your Medical Consent Forms Up to Task? Don’t Leave Out These Essential Fields
Key Takeaways
- Medical release forms are essential for secure and legal information sharing.
- Digital forms improve efficiency and enhance data security.
- Common mistakes, like missing details and vague descriptions, can lead to legal issues.
- Consistent updates ensure forms meet current legal standards.
Medical release forms are vital for ensuring the secure and legal sharing of patient information. However, providers may overlook some key components that make these forms effective. It’s important to know what to include to maintain compliance and safeguard your patients’ privacy.
Follow these best practices to ensure your HIPAA consent forms are comprehensive and effective.
What to Include in Your HIPAA Consent Forms
To ensure your consent forms are effective and HIPAA-compliant, it’s essential to follow certain best practices. They can help you protect patient privacy, meet legal requirements, and streamline the information-sharing process. Here’s what you need to include:
1. Ensure Clear and Complete Patient Information
Include fields for the patient’s full name, contact details, date of birth, and other relevant identifiers to ensure clarity and accuracy.
Common Mistake: Leaving out essential patient details, leading to identification errors and potential legal issues.
2. Specify Authorized Recipients Accurately
Clearly define who is allowed to receive the patient’s information by including their names, relationship to the patient, and contact details to avoid misunderstandings.
Common Mistake: Failing to specify the authorized recipients, resulting in unauthorized disclosures.
3. Detail the Types of Information to be Shared
Specify what types of medical information can be disclosed, allowing patients to control which aspects of their medical history are shared.
Common Mistake: Using vague descriptions that leave room for misinterpretation about what information can be shared.
4. State the Purpose of Disclosure Clearly
Outline the reasons for sharing the information, such as for insurance claims or specialist consultations, to provide context and ensure appropriate use.
Common Mistake: Not stating the purpose clearly, which can cause confusion and misuse of the information.
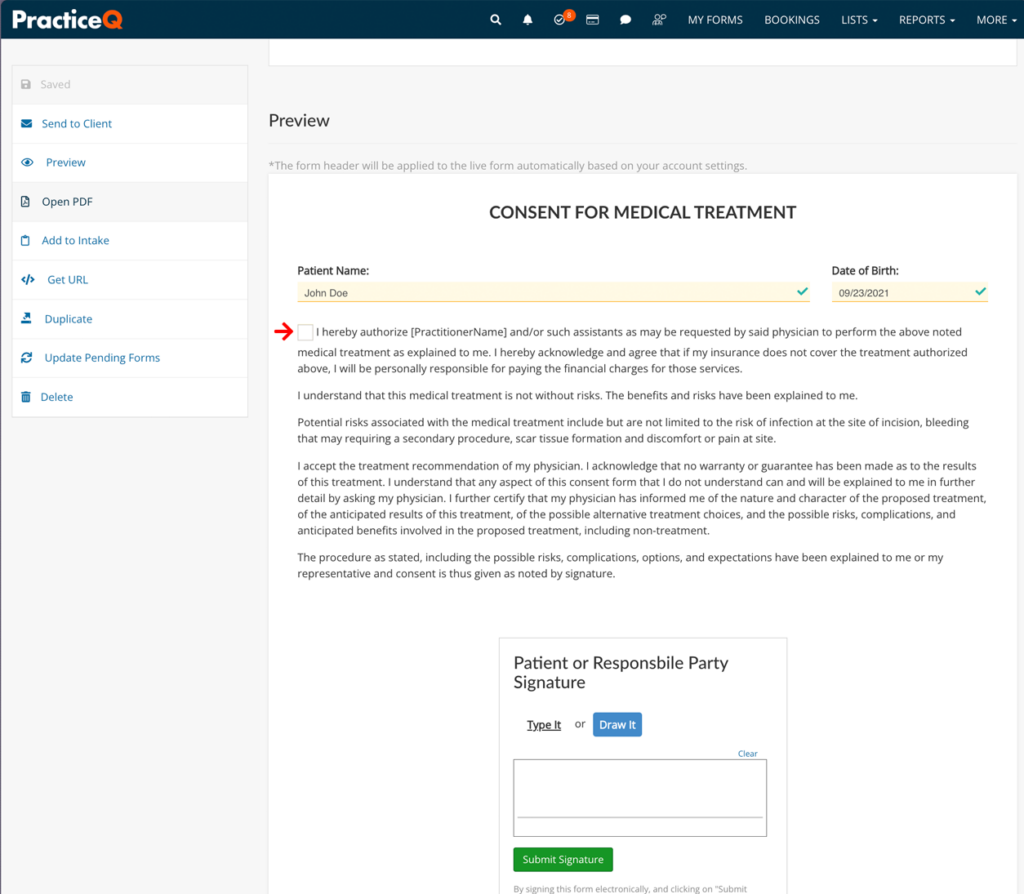
5. Include an Expiration Date or Event
State when the authorization expires, whether by a specific date or an event, to ensure the consent is time-bound and aligns with legal requirements.
Common Mistake: Omitting the expiration date, resulting in open-ended authorizations that increase risk.
6. Provide Legal Disclaimers and Inform Patients of Their Rights
Inform patients of their rights, the process for revoking consent, and any associated fees to maintain transparency and protect both the patient and provider.
Common Mistake: Neglecting to include legal disclaimers, leading to misunderstandings about patient rights and potential disputes.
7. Require a Signature and Date for Formal Consent
Ensure the form is signed and dated by the patient or their representative to formalize the consent and provide a clear record.
Common Mistake: Missing signatures or dates, which can invalidate the consent form.
8. Use Plain Language for Clarity
Write the form in simple, understandable language to avoid confusion and ensure patients fully understand what they’re consenting to.
Common Mistake: Using complex or technical language that confuses patients.
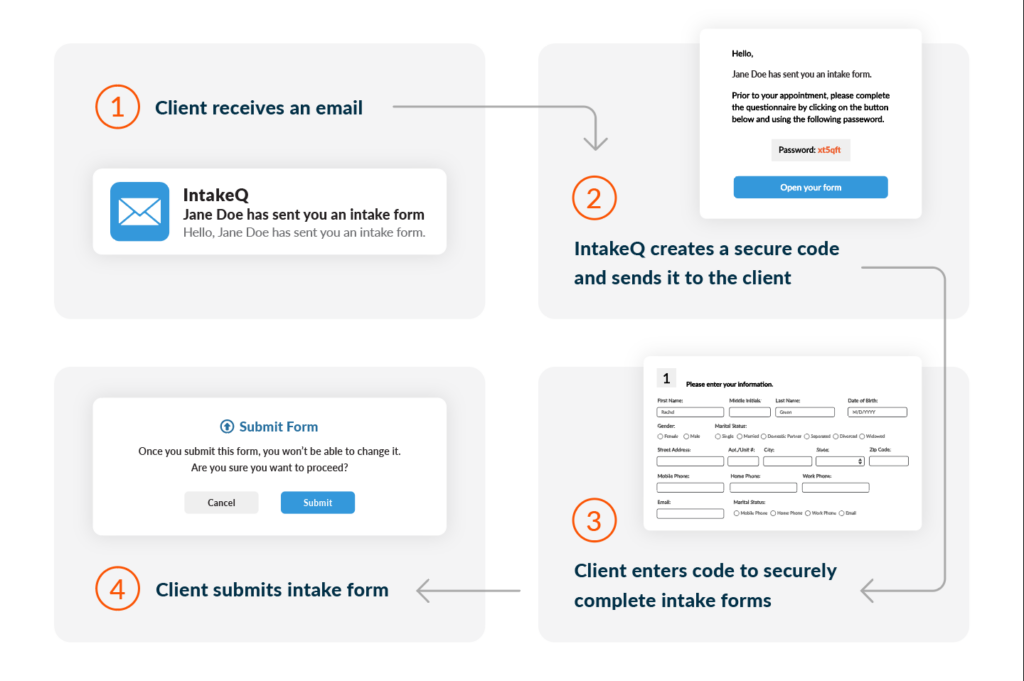
9. Implement Digital Forms for Efficiency and Security
Use electronic forms to streamline the process, enhance security, and provide easy access and tracking of completed forms.
Common Mistake: Sticking to paper forms, which can be less secure and harder to manage.
10. Regularly Update Forms to Comply with Changing Laws and Regulations
Keep the forms up to date with the latest legal requirements and industry standards to ensure ongoing compliance and protection.
Common Mistake: Failing to update forms, leading to non-compliance with current regulations.
11. Verify Patient Competency Before Obtaining Consent
Ensure that the patient is mentally and physically capable of giving consent to avoid legal issues and protect patient rights.
Common Mistake: Obtaining consent from patients who are not competent, which invalidates the form.
12. Review and Update the Forms Regularly
Periodically review and update the forms to address any changes in regulations or practice needs, ensuring they remain comprehensive and effective.
Common Mistake: Neglecting regular reviews, resulting in outdated and potentially non-compliant forms.
Streamline Compliance with HIPAA Compliant Consent Forms
Your healthcare practice strives to stay compliant and provide top-quality care. Don’t let a slip-up on your release forms undo all your hard work. By following these best practices and avoiding common mistakes, you can ensure your forms are comprehensive, reliable, and legally compliant.
For an efficient, secure, and compliant solution, IntakeQ’s digital forms can streamline your process, protect patient information, and maintain the highest standards in your practice. Try IntakeQ today and experience the difference.
Medical Release Form FAQs
What is a medical release form?
A release form (or consent form) is a document that allows healthcare providers to share a patient’s medical information with specified individuals or organizations, ensuring privacy and compliance with laws like HIPAA.
Why are medical release forms necessary?
These forms are crucial for legally and securely sharing patient information, protecting patient privacy, and ensuring that information is only shared with authorized parties.
How long is a medical consent form valid?
The validity of a consent form depends on the specified expiration date or event. Without a specified date, it typically needs to be renewed periodically to remain compliant.
Can a patient revoke their consent after signing a release form?
Yes, patients have the right to revoke their consent at any time. The process for revocation should be clearly outlined in the form’s legal disclaimers.
What are the consequences of not using a proper consent form?
Failing to use a proper release form can lead to unauthorized information sharing, legal liabilities, and non-compliance with regulations like HIPAA, which can result in significant penalties.
How can digital forms improve the medical release process?
Digital forms, like those offered by IntakeQ, streamline the process by enhancing security, ensuring compliance, and providing easy access and tracking. They help maintain high standards of data protection and efficiency in managing patient information.
Looking for More?
For clinics seeking a comprehensive solution, IntakeQ’s sister platform, PracticeQ, offers a customizable practice management system that integrates seamlessly with IntakeQ’s forms. This integration streamlines operations, ensures robust HIPAA compliance, and helps manage the entire patient journey efficiently.
Learn more about PracticeQ and its features.
References
HIPAA Journal. (n.d.). HIPAA Release Form. HIPAA Journal. https://www.hipaajournal.com/hipaa-release-form/
Office for Civil Rights (OCR). (2022, December 28). What is the Difference Between “Consent” and “Authorization” Under the HIPAA Privacy Rule? U.S. Department of Health and Human Services (HHS). https://www.hhs.gov/hipaa/for-professionals/faq/264/what-is-the-difference-between-consent-and-authorization/index.html

